


One of the simplest examples of electrocyclic reactions is thermal ring closure of (1,3Z,5)-Hexatriene to yield 1,3-cyclohexadiene. A similar ring closure reactions of octatrienes show that the electocyclic ring closure reactions are stereospecific: (2E,4Z,6E)-octatriene gives cis-dimethylated cyclohexadiene under thermal conditions while (2E,4Z,6Z)-octatriene yields the trans isomer. Under photochemical conditions, the products have opposite stereochemistry.
The stereoselectivity of electrocyclic reactions can be rationalized based on the symmetry of frontier molecular orbitals. The conservation of orbital symmetry requires that during the reaction, ends of reactive orbitals should overlap in-phase. Under thermal conditions, the reactive orbital is the HOMO, which in hexatriene and octatriene is symmetric. In order to make a favorable in-phase overlap and give a new Sigma bond, the ends of molecule should undergo disrotatory ring closure. Table below shows π molecular orbitals for a plane-symmetric form of (1,3Z,5)-hexatriene; clicking on the image will bring up Virtual Reality Modeling Language models for orbitals.
| VRML Player | Download Cortona to view 3D models | Link |
| Triene |
LUMO. This orbital is important in the photochemical ring closure. Conrotatory movment of terminal =CH2 groups would the two red lobes together and allow favorable orbital overlap such that a new Sigma orbital can form. |
 |
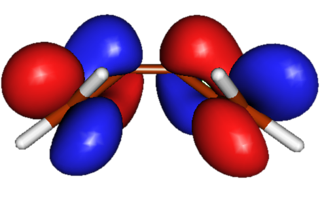
| Triene |
HOMO. This orbital is important in the thermal ring closure reactions. Disrotatory movment of terminal =CH2 groups would bring the two red lobes together and allow favorable orbital overlap such that a new Sigma orbital can form. |
 |
| Triene |
HOMO-1. This orbital rearrangges to become (asymmetric) HOMO in the reaction product (diene). |
 |
| Triene |
HOMO-2. This orbital rearranges to become (symmetric) HOMO-1 in the reaction product (diene). |
 |
Pericyclic Reactions by Frederick E. Ziegler at Yale University
Sakai and Takane: "Theoretical Studies of the Electrocyclic Reaction Mechanisms of Hexatriene to Cyclohexadiene" Research publication in J. Org. Chem., 64, 6842-6848 (1999).